 |
| 1 |  | 
What happens to a hole in a flat metal plate when the plate expands on being heated? Does the hole get larger or smaller? |
|  | |
|
|
 |
| 2 |  | 
Natural gas is sold by volume. In the United States, the price charged is usually per cubic foot. Given the price per cubic foot, what other information would you need in order to calculate the price per mole? |
|  | |
|
|
 |
| 3 |  | 
The temperature at which liquid nitrogen boils (at atmospheric pressure) is 77 K. Express this temperature in (a) °C and (b) °F. |
|  | |
|
|
 |
| 4 |  | 
Steel railroad tracks of length 18.30 m are laid at 10.0°C (Fig. 13.19). How much space should be left between the track sections if they are to just touch when the temperature is 50.0°C?
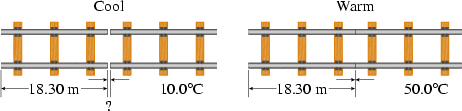 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::Fig. 13.19::/sites/dl/free/0070524076/57996/fig_019.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif">Fig. 13.19 (59.0K)</a>Fig. 13.19 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::Fig. 13.19::/sites/dl/free/0070524076/57996/fig_019.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif">Fig. 13.19 (59.0K)</a>Fig. 13.19 |
|  | |
|
|
 |
| 5 |  | 
A lead rod and a common glass rod both have the same length when at 20.0°C. The lead rod is heated to 50.0°C. To what temperature must the glass rod be heated so that they are again at the same length? |
|  | |
|
|
 |
| 6 |  | 
Find the molecular mass of ammonia (NH3). |
|  | |
|
|
 |
| 7 |  | 
The mass of one mole of 13C (carbon-13) is 13.003 g. (a) What is the mass in u of one 13C atom? (b) What is the mass in kg of one 13C atom? |
|  | |
|
|
 |
| 8 |  | 
How many moles of He are in 13 g of He? |
|  | |
|
|
 |
| 9 |  | 
What is the total translational kinetic energy of the gas molecules of 0.420 mol of air at atmospheric pressure that occupies a volume of 1.00 L (0.0010 m3)? |
|  | |
|
|
 |
| 10 |  | 
What is the total internal kinetic energy of 1.0 mol of an ideal gas at 0.0°C and 1.00 atm? |
|  | |
|
|
 |
| 11 |  | 
What are the rms speeds of helium atoms, and nitrogen, hydrogen, and oxygen molecules at 25°C? |
|  | |
|
|
 |
| 12 |  | 
The reaction rate for the hydrolysis of benzoyl-l-arginine amide by trypsin at 10.0°C is 1.878 times faster than that at 5.0°C. Assuming that the reaction rate is exponential as in Eq. (13-24), what is the activation energy? |
|  | |
|
|
 |
| 13 |  | 
About how long will it take a perfume molecule to diffuse a distance of 5.00 m in one direction in a room if the diffusion constant is 1.00 × 10-5 m2/s? Assume that the air is perfectly still—there are no air currents. |
|  | |
|
|

