Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
Consider the reaction:
CH3Cl(aq) + OH-(aq) →
CH3OH(aq) + Cl-(aq)
If the rate of appearance of CH3OH(aq) remains constant at 1.0 x
10-4 mol/(L·min), and the initial concentrations of both reactants are 0.10 mol/L, how long will it take to consume half of the reactants? |
|  | A) | 10,000 min |
|  | B) | 1,000 min |
|  | C) | 500 min |
|  | D) | 100 min |
|  | E) | none of the above |
 |
 |
2 |  | 
Consider the reaction
2A + 2B + C →
2D + E
If the rate law for this reaction is Rate = k[A][B]2, what will be the effect on the rate if the concentrations of A, B and C are all doubled at the same time? |
|  | A) | The rate will increase by a factor of 2. |
|  | B) | The rate will increase by a factor of 4. |
|  | C) | The rate will increase by a factor of 6. |
|  | D) | The rate will increase by a factor of 8. |
|  | E) | More information is needed before this question can be answered. |
 |
 |
3 |  | 
Nitric oxide (NO) reacts with hydrogen (H2) according to the equation:
2NO(g) + 2H2(g) →
N2(g) + 2H2O(g)
The following initial rates of reaction have been measured for the given reactant concentrations.
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image56.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image56.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> |
Which of the following is the rate law (rate equation) for this reaction? |
|  | A) | rate = k[NO]2 [H2] |
|  | B) | rate = k[NO] [H2]2 |
|  | C) | rate = k[NO] [H2]4 |
|  | D) | rate = k[NO] [H2] |
|  | E) | rate = k[NO]1/2 [H2]1/4 |
 |
 |
4 |  | 
The following initial rate data were collected for the reaction:
2A + B → C + D
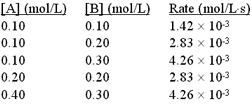 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image57.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image57.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> |
What is the value of the rate constant? |
|  | A) | k = 1.42 x 10-2 s-1 |
|  | B) | k = 2.83 x 10-3 L mol-1 s-1 |
|  | C) | k = 1.42 L2 mol-2 s-1 |
|  | D) | k = 0.532 x 10-3 L2 mol-2 s-1 |
|  | E) | k = 4.26 x 10-1 L mol-1 s-1 |
 |
 |
5 |  | 
A solution of sucrose in water has an initial concentration of 0.155 mol/L. The sucrose is being converted to other sugars in a reaction which is first-order with respect to the sucrose concentration. Exactly 30 minutes later, the sucrose concentration has fallen to 0.131 mol/L. Calculate the value of the rate constant in s-1. |
|  | A) | 9.3 x 10-5 s-1 |
|  | B) | 4.1 x 10-5 s-1 |
|  | C) | 5.6 x 10-3 s-1 |
|  | D) | 1.7 x 10-1 s-1 |
|  | E) | none of the above |
 |
 |
6 |  | 
The reaction A →
products is first-order with respect to A. Which of the following correctly describes a plot of rate data for this reaction? |
|  | A) | [A] plotted vs time gives a straight line of negative slope. |
|  | B) | [A] plotted vs time gives a straight line of positive slope. |
|  | C) | ln[A] plotted vs time gives a straight line of positive slope. |
|  | D) | ln[A] plotted vs time gives a straight line of negative slope. |
|  | E) | 1/[A] plotted vs time gives a straight line of positive slope. |
 |
 |
7 |  | 
Tetrafluoroethene (C2F4) dimerizes at 300º
C to form octafluorocyclobutane (cyclo-C4F8) according to the equation
2C2F4(g) →
cyclo-C4F8(g)
The rate law for the reaction is:
Rate = (0.080 L mol-1 min-1)[C2F4]2
In an experiment where the initial concentration of tetrafluoroethylene is 0.10 mol/L, what concentration remains after 700 min? |
|  | A) | 8.0 X 10-3 mol/L |
|  | B) | 8.0 X 10-4 mol/L |
|  | C) | 2.8 X 10-3 mol/L |
|  | D) | 0.075 mol/L |
|  | E) | 0.015 mol/L |
 |
 |
8 |  | 
For the reaction A →
products, the rate law is Rate = k[A]2. Starting with [A] = 0.100 mol/L, its concentration is found to be 0.0652 mol/L after 600. seconds. What is the value of the rate constant? |
|  | A) | 5.8 x
10-5 L .
mol-1
.s-1
|
|  | B) | 7.1 x
10-4 L .
mol-1
.s-1
|
|  | C) | 3.2 x
10-3 L .
mol-1
.s-1
|
|  | D) | 8.9 x
10-3 L .
mol-1
.s-1
|
|  | E) | none of the above |
 |
 |
9 |  | 
In an enzymatic fermentation, the initial concentration of sugar is 0.16 mol/L; after 10.0 hours the concentration is 0.080 mol/L; after 20.0 hours the concentration is 0.040 mol/L. What is the order of this reaction, and what is the rate constant? |
|  | A) | zero order; 1.67 x
10-6 s-1 |
|  | B) | first order; 1.93 x
10-5 s-1 |
|  | C) | first order; 1.67 x
10-6 s-1 |
|  | D) | second order; 3.71 x
10-10 s-1 |
|  | E) | second order; 1.67 x
10-6 s-1
|
 |
 |
10 |  | 
From the following data, estimate the activation energy for the gas-phase reaction
H2 + I2 →
2HI
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image58.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38225/Image58.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> |
|
|  | A) | 21.0 kJ/mol |
|  | B) | 2.10 x
104 kJ/mol |
|  | C) | 1.75 x
102 kJ/mol |
|  | D) | 9.00 x
102 kJ/mol |
|  | E) | none of the above |
 |
 |
11 |  | 
A second-order rate constant is given by
k = (6.5 x
107 L·mol-1·s-1)e-13500 K/T
What is the the activation energy, Ea, for this reaction? |
|  | A) | 135 kJ/mol |
|  | B) | 56 kJ/mol |
|  | C) | 112 kJ/mol |
|  | D) | 162 kJ/mol |
|  | E) | 782 kJ/mol |
 |
 |
12 |  | 
The reaction of ethylene (C2H4) with butadiene (C4H6) to form cyclohexene (C6H10) has an activation energy (Ea) of 115 kJ/mol. The reverse reaction (decomposition of cyclohexene to ethylene and butadiene) has an activation energy of 287 kJ/mol. What is the heat of reaction, Δ
Hrxn, for the forward reaction? |
|  | A) | +115 kJ/mol |
|  | B) | +287 kJ/mol |
|  | C) | -287 kJ/mol |
|  | D) | +172 kJ/mol |
|  | E) | -172 kJ/mol |
 |
 |
13 |  | 
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution.
(1) H+ + H2O2 →
H3O2+ (rapid equilibrium)
(2) H3O2+ + Br- →
HOBr + H2O (slow)
(3) HOBr + H+ + Br- →
Br2 + H2O (fast)
What is the overall reaction equation for this process? |
|  | A) | 2H+ + H2O2 + Br- + HOBr →
H3O2+ + Br2 + H2O |
|  | B) | H3O2+ + H+ + 2Br- →
Br2 + 2H2O |
|  | C) | 2H+ + 2Br- + H2O2 →
Br2 + 2H2O |
|  | D) | 2H3O2+ + 2Br- →
H2O2 + Br2 + 2H2O |
|  | E) | none of the above |
 |
 |
14 |  | 
The mechanism for the gas-phase reaction
2NO + Cl2 →
2NOCl
is suggested to be:
(1) NO + NO →
N2O4 (slow)
(2) N2O4 + Cl2 → 2NOCl (fast)
Based on this mechanism, the rate law for the overall reaction is: |
|  | A) | Rate = k [NO] |
|  | B) | Rate = k [NO]2 |
|  | C) | Rate = k [NO2]2[Cl2] |
|  | D) | Rate = k [Cl2] |
|  | E) | Rate = k [NO2]2[Cl2]2
|
 |
 |
15 |  | 
At elevated temperatures in the gas phase, cyclopropane reacts to form propene. The rate of reaction is given by:
rate = Δ[propene]/Δt = k[cyclopropane].
Which one of the following actions is least likely to cause a change in the rate of this reaction? |
|  | A) | adding a catalyst |
|  | B) | raising the temperature |
|  | C) | doubling the initial amount of cyclopropane |
|  | D) | halving the volume of the reaction vessel, but keeping the initial amount of cyclopropane constant. |
|  | E) | continuously removing propene as it is formed |
 |

