 |
1 |  | 
What is the proper Lewis structure of HNO3? |
|  | A) | 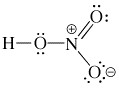 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> |
|  | B) | 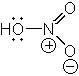 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> |
|  | C) | 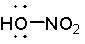 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> |
|  | D) | 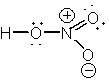 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_D.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_1_D.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> |
 |
 |
2 |  | 
How many lone pairs of electrons will be present in the following molecule?
CH3–N=N=N |
|  | A) | 0 |
|  | B) | 1 |
|  | C) | 2 |
|  | D) | 3 |
 |
 |
3 |  | 
In the nitrate ion (NO3-), what is the charge on nitrogen? All electrons are shown.
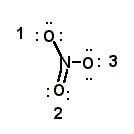 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_3_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_3_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> |
|  | A) | No charge |
|  | B) | - 1 |
|  | C) | + 1 |
|  | D) | + 2 |
 |
 |
4 |  | 
The best Lewis structure for ethyne (C2H2; commonly called “acetylene”) has |
|  | A) | 2 single bonds, 1 triple bond, and 1 lone pair. |
|  | B) | 2 double bonds and 2 lone pairs. |
|  | C) | 2 single bonds, 1 triple bond, and no lone pairs. |
|  | D) | 2 single bonds, 1 double bond, and 2 lone pairs. |
 |
 |
5 |  | 
What is the formal charge (FC) of each atom in the following molecule?
NH4+ |
|  | A) | FC Hydrogens: 0
FC Nitrogen: 0 |
|  | B) | FC Hydrogens: 0
FC Nitrogen: -1 |
|  | C) | FC Hydrogens: 0
FC Nitrogen: +2 |
|  | D) | FC Hydrogens: 0
FC Nitrogen: +1 |
 |
 |
6 |  | 
What is the formal charge (FC) of each atom in the following molecule?
BH4- |
|  | A) | FC Boron: 0
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0
FC Hydrogen4: 0 |
|  | B) | FC Boron: 0
FC Hydrogen1: +1
FC Hydrogen2: -1
FC Hydrogen3: 0
FC Hydrogen4: 0 |
|  | C) | FC Boron: -2
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0
FC Hydrogen4: 0 |
|  | D) | FC Boron: -1
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0
FC Hydrogen4: 0 |
 |
 |
7 |  | 
What is the formal charge (FC) of each atom in the following molecule?
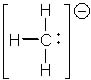 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_6_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_6_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> |
|  | A) | FC Carbon: +1
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0 |
|  | B) | FC Carbon: 0
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0 |
|  | C) | FC Carbon: -2
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0 |
|  | D) | FC Carbon: -1
FC Hydrogen1: 0
FC Hydrogen2: 0
FC Hydrogen3: 0 |
 |
 |
8 |  | 
How many total resonance structures can be drawn for the following anion (include those without separation of charge)?
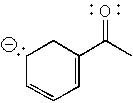 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_7_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_7_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> |
|  | A) | 1 |
|  | B) | 2 |
|  | C) | 3 |
|  | D) | 4 |
 |
 |
9 |  | 
How many resonance structures can be drawn for the following molecule?
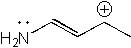 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_9_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_9_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> |
|  | A) | 1 |
|  | B) | 4 |
|  | C) | 3 |
|  | D) | 2 |
 |
 |
10 |  | 
How many other major contributing resonance structures are possible for the following heterocycle?
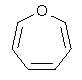 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_10_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_10_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> |
|  | A) | 2 |
|  | B) | 4 |
|  | C) | 6 |
|  | D) | 8 |
 |
 |
11 |  | 
How many energetically equivalent resonance structures exist for the oxalate dianion?
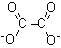 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_11_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_11_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> |
|  | A) | 1 |
|  | B) | 2 |
|  | C) | 3 |
|  | D) | 4 |
 |
 |
12 |  | 
What is the electron configuration for oxygen? |
|  | A) | 1s2 2s2 2p6 |
|  | B) | 1s2 2s2 2p5 |
|  | C) | 1s2 2s2 2p4 |
|  | D) | 1s2 2s2 2p3 |
 |
 |
13 |  | 
Which of the following compounds does NOT have an octet around the central atom? |
|  | A) | NH3 |
|  | B) | BH3 |
|  | C) | H3O+ |
|  | D) | NH4+ |
 |
 |
14 |  | 
Which of the following statements about an sp hybridized carbon is FALSE? |
|  | A) | It is divalent. |
|  | B) | It forms bonds that are linear. |
|  | C) | It has two p orbitals. |
|  | D) | It always forms triple bonds to carbon. |
 |
 |
15 |  | 
A p orbital has what shape? |
|  | A) | an oval |
|  | B) | a sphere |
|  | C) | a wedge of pie |
|  | D) | a dumbbell |
 |
 |
16 |  | 
The hybridization of the central carbon in 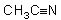 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_16_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> and the bond angle CCN are <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_16_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> and the bond angle CCN are |
|  | A) | sp2, 180°. |
|  | B) | sp, 180°. |
|  | C) | sp2, 120°. |
|  | D) | sp3, 109°. |
 |
 |
17 |  | 
What are the hybridizations of carbons 1 and 2 respectively in the following structure?
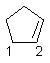 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_17_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_17_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> |
|  | A) | sp3 and sp2 |
|  | B) | sp2 and sp3 |
|  | C) | sp3 and sp |
|  | D) | sp2 and sp2 |
 |
 |
18 |  | 
What are the hybridizations of atoms 1 and 2 respectively in the following structure?
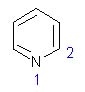 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_18_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_18_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> |
|  | A) | sp3 and sp2 |
|  | B) | sp2 and sp3 |
|  | C) | sp3 and sp |
|  | D) | sp2 and sp2 |
 |
 |
19 |  | 
Identify the orbital hybridization at the two indicated carbons in the molecule below.
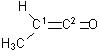 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_19_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_19_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> |
|  | A) | C1: sp; C2: sp |
|  | B) | C1: sp2; C2: sp2 |
|  | C) | C1: sp; C2: sp2 |
|  | D) | C1: sp2; C2: sp |
 |
 |
20 |  | 
The geometric shape of acetone, (CH3)2CO, is best described as |
|  | A) | linear. |
|  | B) | trigonal planar. |
|  | C) | bent. |
|  | D) | tetrahedral. |
 |
 |
21 |  | 
The correct geometry around oxygen in CH3OCH3 is |
|  | A) | linear. |
|  | B) | bent. |
|  | C) | tetrahedral. |
|  | D) | trigonal planar |
 |
 |
22 |  | 
Which molecule has the largest dipole moment? |
|  | A) | HCl |
|  | B) | CCl4 |
|  | C) | H2S |
|  | D) | CO2 |
 |
 |
23 |  | 
Which molecule has the smallest dipole moment? |
|  | A) | CO2 |
|  | B) | CHCl3 |
|  | C) | H2O |
|  | D) | NH3 |
 |
 |
24 |  | 
Consider the structure with the electron-pushing arrow:
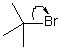 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_24_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_24_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a>
Which of the following statements is FALSE? |
|  | A) | The bond between the tertiary carbon and bromine is breaking. |
|  | B) | The products will be a carbon cation and bromine anion. |
|  | C) | This process is called homolytic cleavage. |
|  | D) | The C–Br bonding electrons become a lone pair on the bromine. |
 |
 |
25 |  | 
What is the product of the curved arrow mechanism shown below?
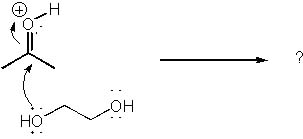 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_eq.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> |
|  | A) | 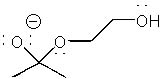 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> |
|  | B) | No reaction. |
|  | C) | 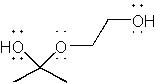 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> |
|  | D) | 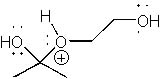 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_25_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> |
 |
 |
26 |  | 
In this reaction, HNO3 + H2SO4 → HSO4– + H2NO3+ a curved arrow should point |
|  | A) | from HNO3 to H2SO4. |
|  | B) | from H2SO4 to HNO3. |
|  | C) | from HSO4– to H2NO3+. |
|  | D) | from H2NO3+ to HSO4–. |
 |
 |
27 |  | 
For the protonation of acetone, the correct curved arrow mechanism is |
|  | A) | 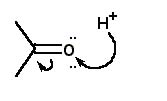 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_A.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> |
|  | B) | 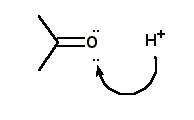 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_B.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> |
|  | C) | 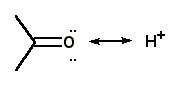 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_C.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> |
|  | D) | 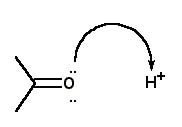 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_D.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072397462/209329/1_27_D.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> |
 |

