 |
1 |  | 
How are MCLG and MCL values related for a carcinogenic drinking water pollutant such as benzene? |
|  | A) | By law, MCLG and MCL values must both be set to zero. |
|  | B) | By law, the MCL values are regularly lowered, making it impossible to identify any common relationship between the MCLG and MCL values |
|  | C) | By law, the MCL value will be set to zero, but the MCLG value will take into account the technical and financial barriers to meeting health goals, so will typically be somewhat higher. |
|  | D) | By law, the MCLG value will be set to zero, but the MCL value will take into account the technical and financial barriers to meeting health goals, so will typically be somewhat higher. |
 |
 |
2 |  | 
How is the cleaning ability of soap to remove grease from clothes related to its structure? |
|  | A) | The long hydrocarbon chain is nonpolar and will dissolve readily in polar grease. The negatively charged end of the "soap ion" will stick out of the surface of the grease globule and will disperse in water long enough to get carried away with the rinse water. |
|  | B) | The long hydrocarbon chain is polar and will dissolve readily in polar grease. The negatively charged end of the "soap ion" will stick out of the surface of the grease globule and will disperse in water long enough to get carried away with the rinse water. |
|  | C) | The long hydrocarbon chain is nonpolar and will dissolve readily in nonpolar grease. The negatively charged end of the "soap ion" will stick out of the surface of the grease globule and will disperse in water long enough to get carried away with the rinse water. |
|  | D) | The long hydrocarbon chain is polar and will dissolve readily in polar water. The negatively charged end of the "soap ion" will stick out of the surface of the grease globule and will disperse in water long enough to get carried away with the rinse water. |
 |
 |
3 |  | 
What is an aquifer? |
|  | A) | An aquifer is a great pool of water trapped below the surface in sand and gravel. |
|  | B) | An aquifer refers to the equipment used by municipal water treatment facilities to purify drinking water. |
|  | C) | An aquifer is a collecting basin for recycled water that can be used only for irrigation. |
|  | D) | An aquifer is a restricted area of surface water used as a resource for municipal drinking water. |
 |
 |
4 |  | 
Numbers 1 - 4 are used to identify four different elements. Based on trends within the periodic table, which element is expected to have the greatest tendency to attract bonded electrons?
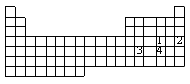 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | element #1 |
|  | B) | element #2 |
|  | C) | element #3 |
|  | D) | element #4 |
 |
 |
5 |  | 
Rank these covalent bonds in order of increasing polarity.
H-N, H-O, and H-S |
|  | A) | H-N, H-S, H-O |
|  | B) | H-O, H-S, H-N |
|  | C) | H-N, H-O, H-S |
|  | D) | H-S, H-N, H-O |
 |
 |
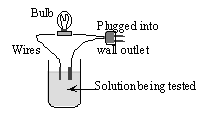
6 |  | 
A 0.1 M solution of ethanol, C2H5OH, in water is tested for conductivity using the type of apparatus shown. What do you predict will happen and why?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | The light bulb will not light up. C2H5OH is not soluble in water. |
|  | B) | The light bulb will not light up. C2H5OH is in molecular form in aqueous solution. |
|  | C) | The light bulb will shine dimly. C2H5OH is only partially ionized in aqueous solution. |
|  | D) | The light bulb will shine brightly. C2H5OH is highly ionized in aqueous solution. |
 |
 |
7 |  | 
When sodium nitrate, NaNO3, dissolves in water, |
|  | A) | ions of Na+, N5+, and O2- are separated from the crystalline NaNO3 by the attraction to the polar water molecules. |
|  | B) | ions of Na+ and NO3- are separated from the crystalline NaNO3 by the attraction to the polar water molecules. |
|  | C) | molecules of NaNO3 are attracted to the nonpolar water molecules. |
|  | D) | molecules of NaNO3 are attracted to the polar water molecules. |
 |
 |
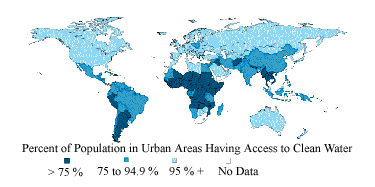
8 |  | 
This world map shows the availability of safe drinking water around the world. Which statement best reflects access to safe drinking water?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a>
|
|  | A) | The vast majority of the urban population of the world has access to safe drinking water. |
|  | B) | Access to safe drinking water is no longer a concern in most parts of the world. |
|  | C) | The urban population of South America and Africa has the greatest access to safe drinking water. |
|  | D) | Access to safe drinking water is common in urban areas of North America. |
 |
 |
9 |  | 
Which is the correct formula and charge for the sulfite ion? |
|  | A) | SO4- |
|  | B) | SO42- |
|  | C) | SO3- |
|  | D) | SO32- |
 |
 |
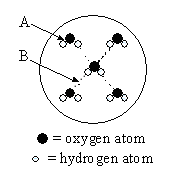
10 |  | 
This diagram represents water molecules arranged in the solid state. Which statement is correct for this diagram?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51619/5A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | Arrow A points to a "hydrogen bond," a type of intermolecular force. Arrow B points to a covalent bond, a type of intramolecular force. |
|  | B) | Arrow A points to a covalent bond, a type of intermolecular force. Arrow B points to a "hydrogen bond," a type of intramolecular force. |
|  | C) | Arrow A points to a "hydrogen bond," a type of intramolecular force. Arrow B points to a covalent bond, a type of intermolecular force. |
|  | D) | Arrow A points to a covalent bond, a type of intramolecular force. Arrow B points to a "hydrogen bond," a type of intermolecular force. |
 |
 |
11 |  | 
Which statement about why consumers are buying bottled water is not correct? |
|  | A) | Bottled water, although expensive, is a very convenient way to carry drinking water for many consumers. |
|  | B) | Some bottled water has a better taste than tap water, because it has been disinfected using ozone or UV radiation, rather than with chlorine. |
|  | C) | The Clean Water Act of 1972 regulates the purity of bottled water, so consumers can be confident that the water is safe to drink. |
|  | D) | Currently more than 3.1 billion gallons of bottled water are sold per year in the U.S., with 18 to 24 year-olds being the largest consumers. |
 |
 |
12 |  | 
Which statement is true for a chloride ion? |
|  | A) | number of protons = 18, number of electrons = 11, net charge = 1+ |
|  | B) | number of protons = 17, number of electrons = 18, net charge = 1- |
|  | C) | number of protons = 16, number of electrons = 17, net charge = 1- |
|  | D) | number of protons = 17, number of electrons = 16, net charge = 1+ |
 |
 |
13 |  | 
Water in many cities is considered "hard." Which ions are responsible for that designation? |
|  | A) | stearate or soap ions |
|  | B) | NaCO3, borax, phosphate |
|  | C) | Ca, Mg, Fe salts of bicarbonate and sulfate |
|  | D) | All of these ions are responsible for a "hard" water designation. |
 |
 |
14 |  | 
Which water treatment method is widely used for disinfecting municipal water supplies, but is not commonly used for treating bottled water? |
|  | A) | ultraviolet radiation |
|  | B) | chlorination |
|  | C) | ozonation |
|  | D) | distillation |
 |
 |
15 |  | 
Which bond is expected to be most polar? |
|  | A) | C-H |
|  | B) | O-H |
|  | C) | Cl-Cl |
|  | D) | S-H |
 |

