 |
1 |  | 
An exothermic process: |
|  | A) | raises the temperature of one gram of a substance by one degree Celsius. |
|  | B) | takes in heat from the surroundings |
|  | C) | increases the acidity of the surroundings |
|  | D) | gives off heat to the surroundings |
|  | E) | releases carbon dioxide into the surroundings |
 |
 |
2 |  | 
Calculate the amount of heat necessary to raise the temperature of 8.5 kg of water from 12.5°C to 84°C |
|  | A) | 2.99 x 103 kJ |
|  | B) | 35.56 J |
|  | C) | 2.54 x 103 kJ |
|  | D) | 2.54 x 106 kJ |
|  | E) | 254 kJ |
 |
 |
3 |  | 
How many calories are required to heat 4.5 g of water from 23 to 85.0 °C |
|  | A) | 383 cal |
|  | B) | 104 cal |
|  | C) | 243 cal |
|  | D) | 279 cal |
 |
 |
4 |  | 
Consider the following exothermic reaction, the rate law at 298 K is: Rate = k [H2] [I2] ----------- H2(g) + I2(g) --> 2 HI(g) Addition of a catalyst would effect the initial rate of the reaction by: |
|  | A) | increasing the initial rate of the forward reaction |
|  | B) | increasing the initial rate of both forward and reverse reactions |
|  | C) | increasing the initial rate of the reverse reaction |
|  | D) | causing no increase or decrease in the rate of reaction |
|  | E) | none of the above |
 |
 |
5 |  | 
Below is the reaction profile for the reaction of (C2H4) with (C4H6). 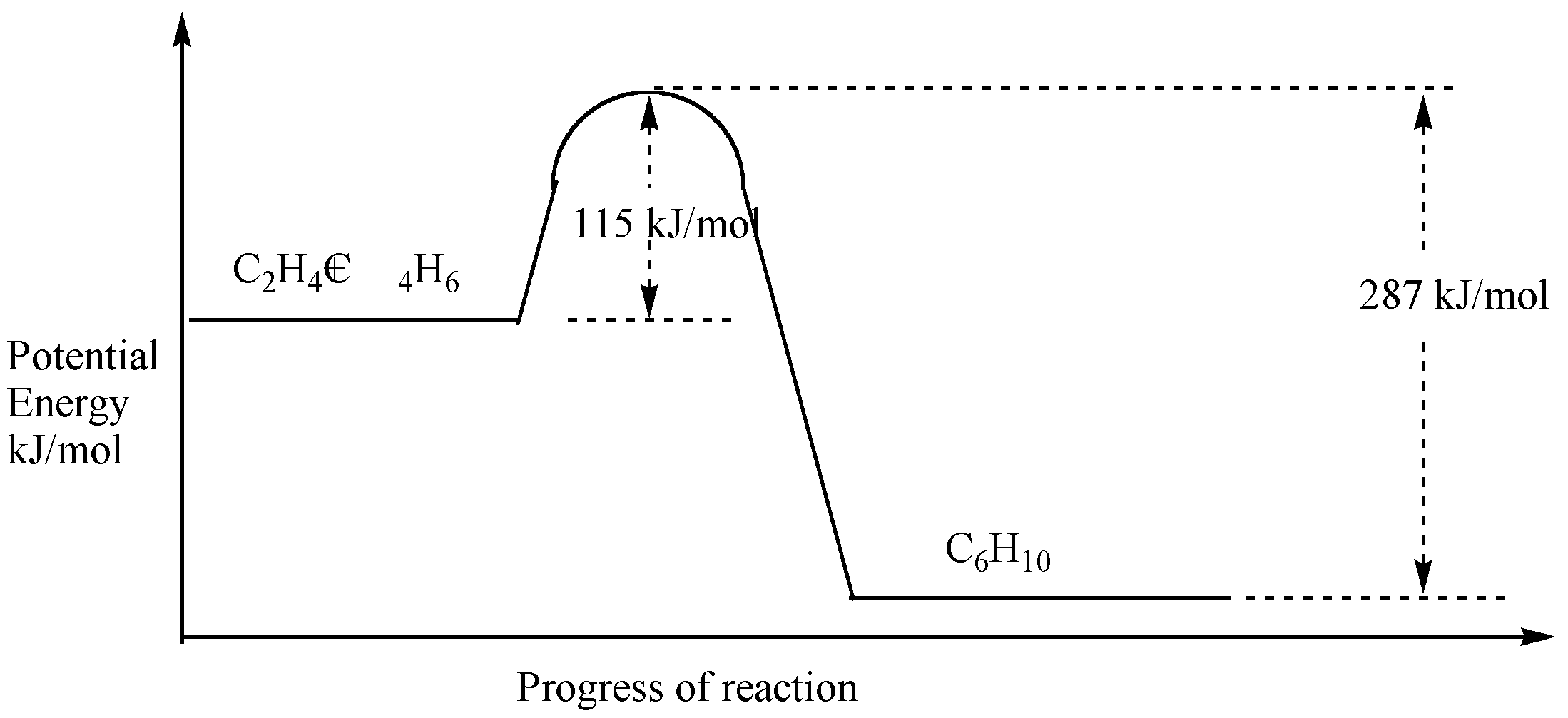 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072920033/80712/image93.gif','popWin', 'width=480,height=267,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (19.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072920033/80712/image93.gif','popWin', 'width=480,height=267,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (19.0K)</a>
Your best estimate for the enthalpy of reaction ()Hrxn) is: |
|  | A) | +115 kJ/mol |
|  | B) | -287 kJ/mol |
|  | C) | +287 kJ/mol |
|  | D) | -172 kJ/mol |
|  | E) | +172 kJ/mol |
 |
 |
6 |  | 
What is the correct equilibrium expression for the following reaction? 2NO2(g) ---> 2NO(g) +O2(g) |
|  | A) | Keq = [O2]2[NO]/[NO2]2 |
|  | B) | Keq = [O2][NO]2/[2NO2]2 |
|  | C) | Keq = [NO2]2/[NO]2[O2] |
|  | D) | Keq = [NO2]2/[NO][O2]2 |
|  | E) | None of the above |
 |
 |
7 |  | 
Which of the following factors will cause the equilibrium 2NOBr(g) ---> 2NO(g) + Br2(g) , H = -47.3 kJ/mol to be shifted to the left (in favor of reactants)? |
|  | A) | Some Ar(g) is added to increase the total pressure in the vessel |
|  | B) | NOBr(g) is added |
|  | C) | A catalyst is added |
|  | D) | The volume of the container is increased to double times its original volume |
|  | E) | The temperature is increased |
 |
 |
8 |  | 
Which of the following factors will cause the equilibrium H2(g) + I2(g) ---> 2HI(g) , H = +15 kJ/mol to be shifted to the left (in favor of reactants)? |
|  | A) | HI(g) is removed. |
|  | B) | I2(g) is added |
|  | C) | Some N2(g) is added to increase the pressure in the vessel |
|  | D) | The volume of the container is reduced to half of its original volume |
|  | E) | The temperature is decreased |
 |
 |
9 |  | 
K is a constant for a certain reaction, but if _____ changes, K will change. |
|  | A) | Pressure |
|  | B) | Time |
|  | C) | Volume |
|  | D) | Temperature |
|  | E) | Concentration |
 |
 |
10 |  | 
Which of the following has the greatest entropy per mole? |
|  | A) | ice |
|  | B) | water |
|  | C) | steam |
|  | D) | all have the same entropy, because all three are H2O |
 |

